Is "disposable mobile phone battery" reliable?
- Share
- Issue Time
- Jul 12,2022
Summary
Question: Is this disposable mobile phone battery reliable? What is its rationale?
"Foreign designers have recently been working on a disposable mobile phone battery, which has won an award. At present, the battery is divided into 2 hours, 4 hours and 6 hours. If there is no accident, it is likely to be available in convenience stores in the future. No longer afraid of going out and running out of battery!"
Is such a small battery enough for power?



The disposable battery, called Mini Power, is the work of designer Tsung Chih-Hsien and won the 2014 Red Dot Design Award.
As mentioned in a report, the principle of this battery is paper battery technology.
As for what a paper battery is, it looks like this (picture from bbc):
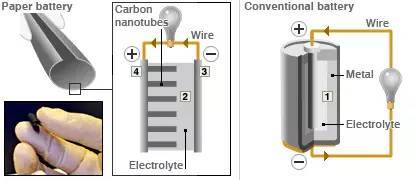
The two schematic diagrams on the left are paper batteries (the picture on the right is a traditional battery)
This kind of battery looks and feels the same as normal paper (because the main component of the material is cellulose - -). Then this "paper" is soaked with ionic liquid (probably paper soaked with ionic liquid?) as the electrolyte (electrolyte in the picture); the electrodes in the paper are carbon nanotubes (2 in the picture), each carbon nanotube The thickness of the tube is about one millionth of a centimeter. A chemical reaction then occurs between the nanotubes and the electrolyte.
This so-called "paper battery" is not a battery in principle, but a Supercapacitor, which is a supercapacitor or an electric double layer capacitor in Chinese.
Someone has designed a supercapacitor electrode of another carbon material. The surface area of the supercapacitor electrode needs to be large in order to hold more electricity. But it seems that so far, there has been no breakthrough in the surface area of carbon nanotube materials.
Compared with batteries, the biggest disadvantage of supercapacitors is that the energy density is much smaller. I read the PNAS literature, the energy density is 13 Wh/kg, and the general lithium-ion battery is over 100, so it looks like 1/8.
That is to say, if the iPhone battery can be used for 6 hours, the weight of the supercapacitor that can supply the iPhone for 6 hours is 8 times that of the original battery. The algorithm here may be to calculate the theoretical time of the iPhone battery. Up to 10 days (250 hours) of standby time on the iPhone 6 and 16 days (384 hours) on the iPhone 6 Plus.
The iPhone 6 battery can be played continuously for about 6 hours under the 4G network. If you convert it, the 2HR in the picture is enough to play for about 3 minutes.